The 2017 Annual Report on Information Disclosure
This annual report is prepared pursuant to the related regulations of Government Information Disclosure Ordinance of the People's Republic of China and based on the actual condition of information disclosures made by Center for Food and Drug Inspection (CFDI) of China Food and Drug Administration in 2017. This report consists of five parts: overview, positive information disclosure, application-based disclosure, information disclosure consultation, and problems in information disclosure and improvement measures. The statistical period for the data presented in this report is from 1 January 2017 to 31 December 2017.
Please don’t hesitate to contact Division of Quality Management of CFDI in case of any doubt. (Address: F6 Bei Kuang Financial Building, No. 1 Wenxing Street, Xicheng District, Beijing. Postcode: 100044. Tel: 010-68441197)
I. Overview
In 2017, the Center for Food and Drug Inspection of CFDA acted conscientiously according to the spirits of the 19th National Congress of the Communist Party of China, followed the guidance by Xi Jinping’s socialist thoughts with Chinese characteristics in the new era, and attached great importance to governmental information disclosure. In accordance with the requirements of the Information Disclosure Regulations of the People’s Republic of China, the Notice of the General Office of the State Council Regarding Printing and Distributing the Work Points of Government Affairs Disclosure (GBF [2017] No.24) , and the 2017 Implementation Plan of Government Affairs Disclosure , CFDI further strengthened the efforts to make relevant information public, took full advantage of multiple channels like Chinese website, English website, and official account to promptly issue a variety of inspection information. Meanwhile, it established additional information disclosure columns and related databases to offer more released information, to protect the right to be informed and to participate for the public and the pharmaceutical industry. In order to standardize release procedure on the website and official account and to ensure that information is published accurately, timely, effectively, and in a standardized manner, it revised the Management Procedure for Website Information Release and the Management Procedure for Information Release on the Official Account .
II. Construction of Information Disclosure Platform
1. Chinese website
In 2017, the Chinese website adjusted and enriched its columns. A total of 7 columns (sub-columns) were added, including 3 hot topics (ICH guidelines, periodical reports on the drug clinical trial data inspection, and dynamic study of international drug inspection), 1 column (consistency assessment), 2 sub-columns (GCP policies and regulations for medical devices, study and implementation of the spirits of the 19th National Congress of the Communist Party of China). In addition, 9 new information disclosure databases were established, including the on-site inspection for one-time qualification verification of vaccine clinical trial institutions, the on-site inspection for registration of vaccine clinical trials, the progress query database for on-site verification of clinical drug trial data, the database for the advance notice of the current month’s inspection, and the database for the notice of the previous month’s inspection. As of December 31, 2017, the Chinese website had established 12 information release columns, including 6 inspection columns and 1 interactive communication column; the announcement column published 14 types of information, and the data query column provided 5 announcement query databases, 6 progress query databases, 4 issues for online reporting and submission, and 1 online application database. In 2017, the views of the Chinese website approximated 3.07 million.

2. English website
The English website of CFDI formally went alive on December 6, 2016. The English website includes “About CFDI”, “Photo News”, “Hot Topics”, “What's New”, “Laws & Regulations”, and “Normative Documents & Guidance”As of December 31, 2017, the website released a total of 161 messages, including 89 messages in “What's New”, 44 in “Hot Topics”, 18 in “Photo News”, and 10 in the remaining columns. In 2017, the English website recorded about 100,000 views.
3. WeChat official account.
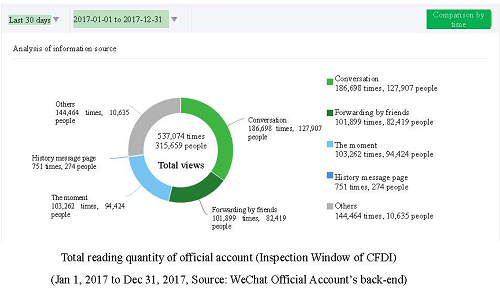
In 2017, the WeChat official account, called “Inspection Window of CFDI”, added two columns, i.e. Inspection Research (under Inspection Information) and Seek for Opinions (under Supervision Information). As of December 31, 2017, the account had established 15 columns in three major sections. In 2017, the total reading quantity of official account were about 540,000.

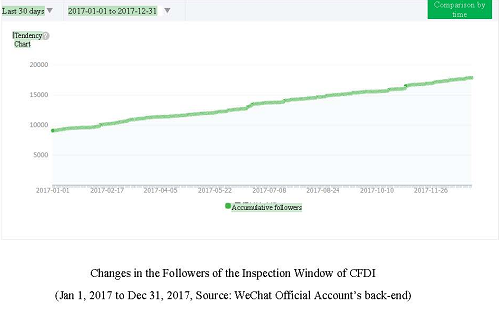
As of December 31, 2017, the followers of the Inspection Window of CFDI totaled 17,860.
III. Positive Information Disclosure
(1) Overall Situation
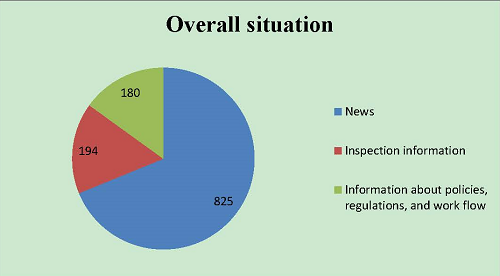
From January 1 to December 31, 2017, the Chinese website positively disclosed 1,199 messages, up by 46% from 2016.In particular, there were 825 pieces of news (68.8%), 194 messages about varied inspection information (16.2%), and 180 messages about policies, regulations, and work flow (15%).
(2) Information Disclosure about Key Areas
In 2017, due to the decentralization of drug GMP certification, less announcement and publicity information about key areas was released on the website.22 pieces of announcement and publicity information were released in total in 2017, including 6 announcements about drug GMP certification, 5 announcements about qualification verification of drug clinical trial institutions, 3 publications about GMP certification review, and 8 announcements about the plan for on-site inspection of drug clinical trial data. It was released that the 2016 Drug Inspection Report (Chinese and English version) and periodical reports on the drug clinical trial data inspection (from July 2015 to June 2017). The columns of Clinical Trial Data Inspection, Consistency Evaluation, Flight/Tracking Inspection, and Overseas Inspection released and forwarded 183 pieces of popular information from the CFDA, an increase of 161% compared with 2016.
(3) Inspection on the construction and maintenance of related databases
As of December 31, 2017, the CFDI's website positively made available to the public 5 announcement query databases providing 23,302 pieces of data including 20,588 announcements about drug GMP certification, 176 announcements about GAP inspection on Chinese medicine materials, 107 announcements about drug GLP certification, 856 announcements about qualification certification for clinical drug trials, and 1,575 publications about drug GMP certification review.
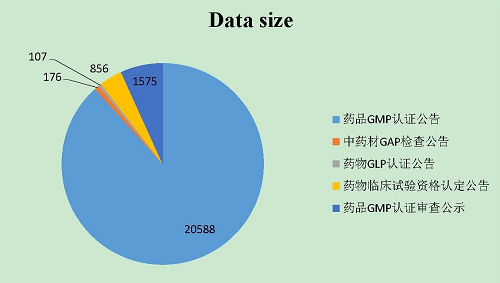
(4) Increase in information releases
As of December 31, 2017, the WeChat office account “the Inspection Window of CFDI” pushed data 192 times and released 405 pieces of information. In particular, there were 204 pieces of information about Supervision and Punishment, 71 for Inspection News, 50 for Inspection Research, 27 for Inspection Announcements, 26 for Seeking for Public Opinions, 10 for inspection plans, 8 for Supervisory Policies, 5 for Technical Guidelines, and 3 for Annual Report.
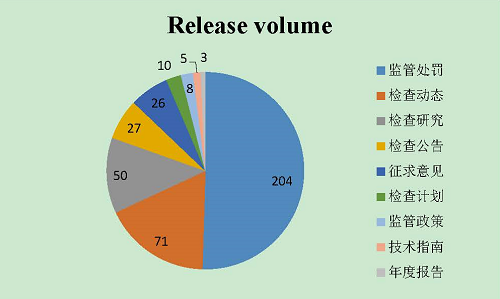
IV. Online Consultation, Complaints and Reply
(1) Online Consultation and Reply
Throughout 2017, a total of 492 online questions were received, of which 421 were valid, down by 32% compared to 2016. The main reason is the changes of the CFDI’s functions and larger degree of information disclosure. Among the valid consultations, 414 were responded to, with response rate as 98%. Among them, 342 involved common problems (or those of high concern) relating to aspects like drug production and had been published on the website. For 72 common questions, replies were provided by email or SMS.
(2) Online Complaints and Handling
Throughout 2017, CFDI received 33 complaints, 28 of which were valid, a decrease of 33% compared with 2016; most invalid complaints were repeated ones, or they were obscurely described and could not be handled. Among invalid complaints, 23 did not fall within the CFDI's functions. They have been transferred to the Center of Complaint and Reporting of CFDA, and 5 cases involved the issues subject to the investigation by the CFDI have been transferred to the CFDI’s relevant business departments for processing. The handling rate of complaints and reported issues was 100%.
V. Main Problems and Improvement Measures
In 2017, despite that CFDI further strengthened information disclosure, released more information through multiple channels, and improved the process of information disclosure, there was still a gap from the goal of comprehensive discourse of inspection information. The content and scope of information disclosure needs to be further expanded. Additionally, the information disclosure mechanism entails further improvement. In 2018, CFDI will earnestly implement the requirements of the Information Disclosure Regulations of the People ’ s Republic of China and superior department, enhance positive disclosure and inspection on disclosure degree, expand disclosure coverage, and pursue more timely disclosure. Meanwhile, CFDI will further strengthen the establishment of relevant systems for information disclosure and strive to improve the quality and level of information disclosure.
Appendix:
![]() Statistical Table of 2017 Annual Information Disclosure.docx
Statistical Table of 2017 Annual Information Disclosure.docx
The former Center for Food and Drug Inspection of CFDA (interim)
April 4, 2018

