2018 Annual Report on Information Disclosure
According to the relevant provisions of the Regulation of the People's Republic of China on the Disclosure of Government Information , this annual report is prepared based on the actual information disclosure situation of the Center for Food and Drug Inspection of NMPA in 2018. The report includes the general situation, the construction of the information disclosure platform, the active disclosure of information, the handling of consultations and complaints, major issues and improvement measures. The statistical period of the data included in this report is from January 1, 2018 to December 31, 2018.
If you have any questions about this report, please contact the Information Management Office of the Center for Food and Drug Inspection of NMPA (Address: 7/F, Bei Kuang Financial Building, No. 1, Wenxing Street, Xicheng District, Beijing; postcode: 100044; Tel: 010-68441197).
1. Overview
In 2018, CFDI, guided by Xi Jinping's Thought on Socialism with Chinese Characteristics for a New Era, conscientiously implemented the spirit of the 19th CPC National Congress, attached great importance to the information disclosure work. According to the requirements of the Regulation of the People's Republic of China on the Disclosure of Government Information and the Implementation Plan for Disclosure of Administrative Affairs of NMPA in 2018 and adhered to the purpose of improving services, enhanced the network and information security, further strengthened the institution construction, completed the active disclosure catalogue, highlighted the disclosure of information in key areas, and made efforts to ensure the information disclosure work on pertinence, timeliness and effectiveness.
2. Construction of information disclosure platform
According to the Guidelines for Development of Government Websites and the Opinions of the China Food and Drug Administration on the Implementation of the Guidelines for Development of Government Websites , CFDI strengthened the adjustment and optimization of the website columns to ensure the timeliness and accuracy of the information disclosure. CFDI gave full play to various information disclosure platforms and channels and established multiple information disclosure platforms such as the Chinese website, the English website, WeChat Official Account, Exhibition system, etc. to actively disclose information through multiple channels. In the NMPA's first evaluation on the websites of its directly affiliated institutions in 2018, CFDI ranked the 4th in terms of total score. Among the six special evaluation indicators, the score of information release and interactive communication ranked the first while that of web page standardization degree and innovation development ranked the second.
(1) Chinese and English websites
As of December 31, 2018, a total of 32 columns had been created on the Chinese website, including 5 dynamic news items, 3 bulletin information columns, 1 policy and regulation column, 2 data query columns, 12 special topic columns, 1 institutional function column, 2 interactive communication columns, and 6 service columns. The English website has 6 columns, including 3 dynamic news columns, 1 institutional function column, and 2 policy and regulation columns.
The homepage of CFDI website
The homepage of the English website
In order to implement the Notice on Strengthening the On-site Inspection on the Registration Application of Chemical Generic Injections (No. 20 [2018]), strengthen the drug injection review and approval, and ensure the drug safety and effectiveness, CFDI carried out the On-site Inspection on the Registration Application of Chemical Generics Injections In 2018. In order to cope with this work, the information about the on-site inspection on the registration production of chemical generic injections was added in the service column of the website to facilitate the registration applicants to file an application and fill in information on line.
In 2018, the Chinese and English websites were visited for 3.64 million times, an increase of 15% over 2017.
(2) WeChat Official Account
As of December 31, 2018, the WeChat official account titled “the Window of CFDI Inspection and Verification” had established 3 sections composed of 15 columns, including 1 dynamic news column, 3 bulletin information columns, 3 policy and regulation columns, 4 special topic columns, 3 institutional function columns and 1 service column. The WeChat official account went on line on December 6, 2016. As of December 31, 2018, the number of its fans reached 25,000. In 2018, the number of its fans increased by 7,140.

WeChat official account 's QR code
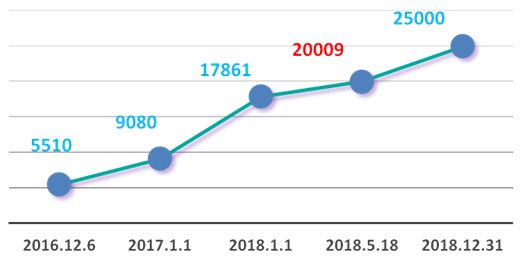
The number of fans of the WeChat official account
(3) Exhibition system
In order to promote the disclosure of the government affairs, strengthen the handy service for the public, and accept the social supervision, In 2018, CFDI organized and developed exhibition system which was installed in the display and inquiry machine in the reception area to facilitate the visitors to learn about the work procedure and content of CFDI and prepare relevant procedures and materials. The exhibition content includes the service guide, introduction to CFDI, information disclosure and the party building propaganda. The related content is synchronized with the website and updated in real time.
3. Active disclosure of information
(1) Further improve the relevant institution
CFDI revised and published the Work Information Management Measures and the Procedures for Information Release and Management on WeChat Official Account to regulate the management of the information collection, entry and use processes to ensure the accurate, timely and effective of information release.
(2) Strengthen active disclosure
From January 1st to December 31st, 2018, the Chinese website totally disclosed 1,302 pieces of information, including 856 pieces of news and announcement information, 391 pieces of information about inspection work, 6 pieces of information about policies and regulations and 49 pieces of other information. The English website published 51 pieces of information.
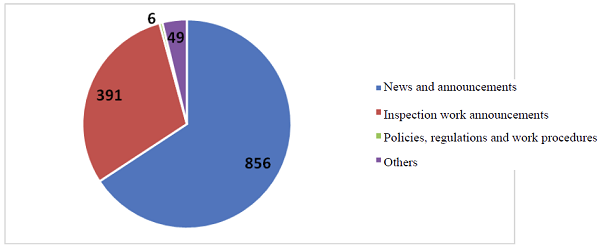
The overall situation of information disclosure on the website of the CFDI
(3) Strengthen the information disclosure in key areas
In 2018, a total of 16 public announcements were released, including 1 Good Laboratory Practice verification announcement, 2 clinical drug trial institution qualification affirmation announcements, 3 clinical drug trial institution qualification review announcements, 1 drug GMP certification review announcement, and 9 announcements on the on-site inspection plan for clinical drug trial data. The 2017 drug inspection report (in both Chinese and English) was issued. The clinical trial data verification column, the consistency evaluation column, the unannounced/tracking inspection column, and the overseas inspection column released and forwarded a total of 364 pieces of hot spot information related to the CFDA, representing an increase of 99% over 2017.
A total of 1,234 on-site inspection/verification advance notices and announcements, including 304 advance notices and announcements on on-site inspection of drug registration production, 68 advance notices and announcements on GLP on-site inspection, 228 advance notices and announcements on on-site inspection of clinical drug trial institution qualification affirmation, 580 advance notices and announcements on on-site inspection of clinical drug trial data, 54 advance notices and announcements on on-site inspection of one-off vaccine clinical trial institution qualification affirmation.
(4) Steadily promote the construction and maintenance of the query database
As of December 31, 2018, CFDI totally opened 5 announcement inquiry databases to the public, with a data volume of 23,427, including 20,588 GMP certification announcements, 176 TCM GAP inspection announcements, 117 GLP certification announcements, 970 clinical drug trial qualification affirmation inspection announcements; and 1,576 GMP certification review data announcements.
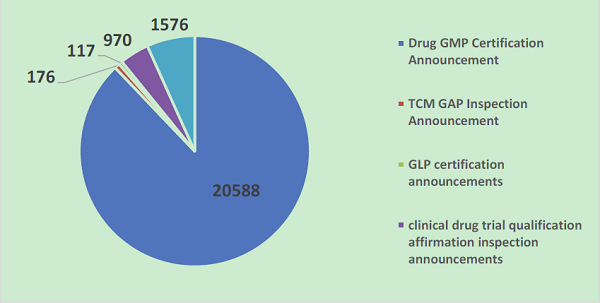
Data volume of various databases
(5) Continue to expand the volume of information released through the WeChat official account
As of December 31, 2018, the official account had pushed information for 366 times, and released 892 pieces of information, with the total number of readers reaching 662,271 and the number of times of view 1,192,554. In 2018, the official account pushed information for 174 times, and released 485 pieces of information, with the total number of readers reaching 306,655 and the number of times of view 592,431.
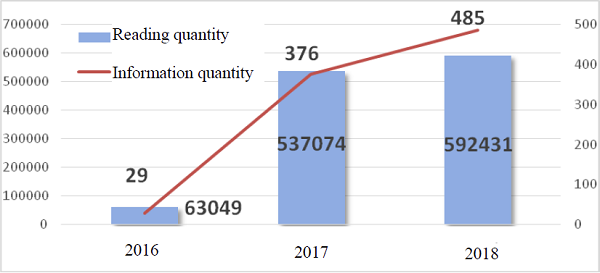
WeChat official account's annual information release volume and reading quantity
Classified by the information column, the numbers of pieces of information released in main columns in 2018 are as follows: 319 pieces of information about regulatory penalties, 60 pieces inspection dynamics, 39 pieces about inspection studies, 25 pieces about inspection announcements, 12 pieces about regulatory policies, 11 pieces about seeking for opinions, 9 pieces about inspection plans, 6 pieces about technical guidelines and 3 pieces about annual reports
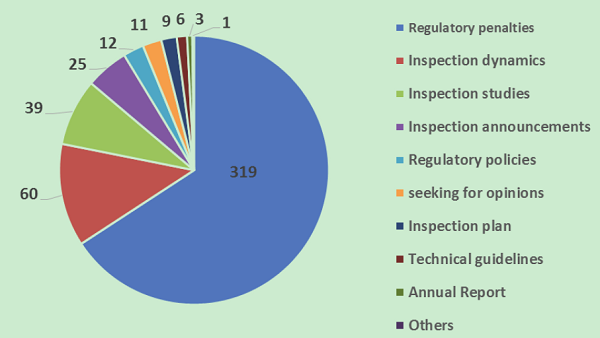
Information release volume of various columns of the WeChat official account
4. Handling of consultations and complaints
(1) On-line consultations
In 2018, a total of 744 on-line consultations were received, including 669 valid consultations, up 60% over 2017, and 75 invalid consultations due to incomplete or unclear description. A total of 317 valid consultations were answered, representing a response rate of 47%, of which 88 consultations related to pharmaceutical production or other common problems (or problems with great attention) were released on the website and 229 consultations about general problems were answered by email or SMS.
(2) On-line complaints and handling
In 2018, a total of 48 complaints were received, including 35 valid complaints, up 25% over 2017 and 13 invalid complaints. Most of invalid complaints were repeated complaints or included unclear description. Among the valid complaints, 23 complaints were out of the functional scope of CFDI and have been transferred to NMPA Administrative Affairs Acceptance Service and Complaints Reporting Center and 12 complaints related to CFDI inspections were transferred to the business departments of CFDI for processing. The handling rate of complaints and reports was 100%.
V. Main issues and improvement measures
In 2018, CFDI further strengthened the construction of the information disclosure institution, improved the active disclosure catalog, highlighted the disclosure of information in key areas, and shrived to improve the pertinence, timeliness and effectiveness of the disclosed information. However, the purpose of comprehensive disclosure of inspection information was not achieved yet. The information integrity needs to be improved; the ability to integrate resources needs to be promoted; and the construction of interactive communication channels needs to be completed. In 2019, CFDI will conscientiously implement the spirit of the 19th Party Congress, strictly follow the relevant requirements of the Regulation of the People's Republic of China on the Disclosure of Government Information and the National Medical Products Administration, and further strengthen the drug inspection information disclosure as well as the platform construction. We will focus on the following work:
(1) According to the relevant requirements of the Guidelines for the Development of Government Websites , continue to promote the active disclosure, improve the information release institution; scientifically project the contents of the columns, highlight key information; promptly respond to the on-line consultations and improve the problem response rate.
(2) Strengthen the construction of the interactive communication channels, establish survey and collection columns, and conduct questionnaire surveys or opinions collection activities around the annual key work.
(3) Play the role of new media, continuously improve the influence of WeChat Official Account, further improve the content construction quality of WeChat Official Account, expand the coverage of active disclosure, and enhance the timeliness of the disclosure.
Appendix:
![]() 2018 Information Disclosure Statistics.doc
2018 Information Disclosure Statistics.doc
National Medical Products Administration
Center for Food and Drug Inspection
April 1, 2019


