2020Annual Report on Information Disclosure
In accordance with relevant provisions of the
Regulation of the People's Republic of China on the Disclosure of Government Information
and in combination with actual situations of the information disclosure of CFDI in 2020, this annual report has been formulated. This report covers an overview, construction of online office service platform, system construction, information disclosure in key areas, construction of website and new media platform, consultation and complaints, problems and improvements, and other aspects. The data listed in this report was summarized from January 1, 2020 to December 31, 2020.
If you have any questions about this report, please contact the Department of Information Management of CFDI (Address: Floor 7, Beikuang Finance Building, Building 3, Yard 1, Wenxing Street, Xicheng District, Beijing; Postal code: 100044; Tel: 010-68441191).
I. Overview
In 2020, CFDI innovated the construction of information disclosure platform, integrated service functions, deepened information disclosure in key areas, and constantly improved the quality of information disclosure in accordance with the Regulation of the People's Republic of China on the Disclosure of Government Information and the Evaluation Index of Websites Directly under the National Medical Products Administration in 2020 .
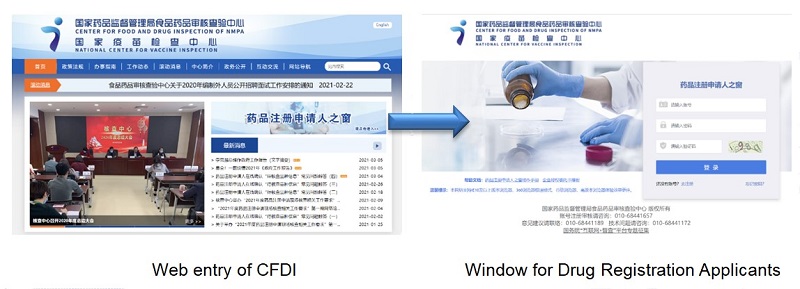
II. Serve drug registration applicants and build a new online office service platform
In order to implement relevant requirements on speeding up the information construction of examination and approval of drugs and medical devices and gradually realizing electronic submissions and evaluation, review and approval of all kinds of applications in the Opinions on Deepening the Reform of Examination and Approval System to Encourage Innovations of Drugs and Medical Devices (T.Z. [2017] No. 42) and Opinions on the Reform of Examination and Approval System for Drugs and Medical Devices (G.F. [2015] No. 44), and to ensure the smooth implementation of the Administrative Measures on Drug Registration , CFDI focused on “serving drug registration applicants” to create a brand new online office service platform - Window for Drug Registration Applicants by combining on-site inspection requirements. The Platform was put into trial operation on July 1, 2020, and officially put into operation on January 1, 2021. As of December 31, 2020, there had been 1,193 users registered. The Platform integrates six inspection categories including on-site production inspection for drug registration, on-site production inspection for registration of generic chemical drugs (injections), on-site development/production inspection for consistency evaluation, and inspection of drug clinical trial data, inspection of clinical trial data for consistency evaluation, pharmacology and toxicology inspection for registration. The function covers notification viewing, progress query, file interchange and query of previous inspections, etc. The application of the “Window for Drug Registration Applicants” has further improved the quality and efficiency of on-site inspections, expanded the information disclosure of on-site inspections, and widened channels for information exchange between CFDI and applicants.
III. Further improve the disclosure of information and construction of management systems
In 2020, CFDI had revised the Procedures for the Website Management , standardized the construction and management of the website and enhanced the information disclosure. Information was disclosed strictly in accordance with the Procedures for information Release and Management on the WeChat Official Account , so as to make sure the information was accurately, timely, standardly and effectively publicized and disclosed.
IV. Intensify information integration and information disclosure of key inspection business
(I) Strengthen the disclosure of information about all kinds of inspections. In 2020, CFDI had issued 2 announcements on inspection plan of clinical trial data of drugs; 145 pieces of thematic information and special column information about on-site inspection plan for registration of generic chemical drugs (injections), on-site inspection plans for pharmaceutical development/production, notices of unannounced inspections of medical devices and notifications on overseas inspection results, and 2699 advance notices and announcements of on-site inspections.
|
Category of Public Announcement Information about Key Fields |
Quantity of Information (pieces) |
Total |
|
1. Public announcement |
|
2 |
|
Announcement on inspection plan of clinical trial data of drugs |
2 |
|
|
2. Thematic information and special column information |
|
145 |
|
Special column for on-site inspections of pharmaceutical development and production |
24 |
|
|
Special column for on-site production inspections for registration of generic chemical drugs (injections) |
6 |
|
|
Special column for medical devices inspections |
18 |
|
|
Special column for overseas inspections |
13 |
|
|
Special column for clinical trial data |
3 |
|
|
Special column for unannounced inspections/trails |
49 |
|
|
Special column for inspection plans |
32 |
|
|
3. Advance notices and announcements for on-site inspections |
|
2699 |
|
On-site inspection of drug registration production |
1367 |
|
|
On-site inspection of GLP drugs |
55 |
|
|
On-site inspection of drug clinical trial data |
1277 |
|
|
Total |
2846 |
|
(II) Release the progress on joint inspections of drug registration. In order to implement the work deployment of the Central Committee of the Communist Party of China and the State Council on coordinating the prevention and control of the epidemic situation and promoting the resumption of production of medical enterprises, according to the requirements of the Notice of Comprehensive Department of NMPA on Doing a Good Job in Inspections for Drug Registration During COVID-19 Epidemic Prevention and Control (Y.J.Z.Y.Z. [2020] No. 15), CFDI had steadily promoted the relevant work of joint inspections for drug registration since March and disclosed the progress of joint inspections on a regular basis since June. In 2020, CFDI had disclosed 7 joint inspections for drug registration, 24 announcements on on-site inspection tasks of pharmaceutical development and production for drug registration applications, and 6 on-site inspection plans for production registration of chemical generic drug- injectable products.
V. Adapt to the new development requirements and continue to promote the construction of websites and new media platforms
1. Establish a special column to publicize “thoroughly studying and implementing well the Xi Jinping Thought on Socialism with Chinese Characteristics for a New Era”, where 61 pieces of information had been disclosed.
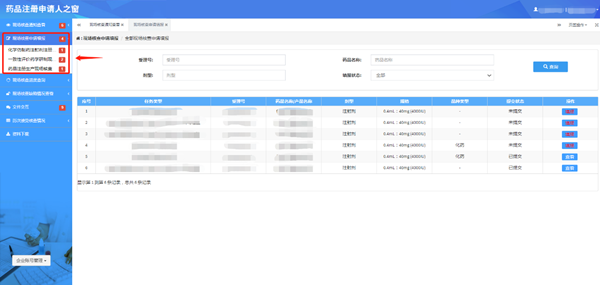
2. Integrate online service columns and optimize on-line service procedures to integrate application submission and information confirmation of on-site production inspection for drug registration, on-site production inspection for registration of generic chemical drugs (injections), on-site development/production inspection for consistency evaluation, and other categories of inspections in the Column “On-line Service-On-line Application” into the Platform “Window for Drug Registration Applicants”.
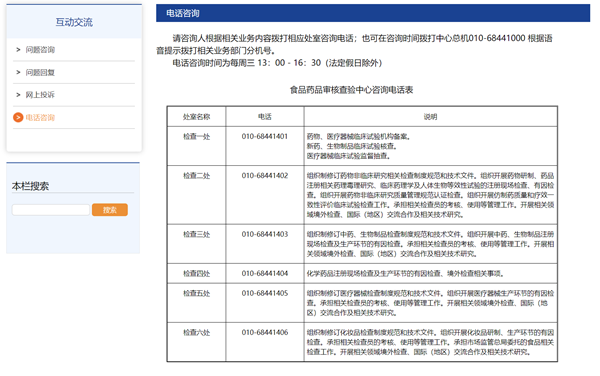
3. Add guidance information for telephone consultation in the interactive exchange column of the Website, including the time for telephone consultation, office telephone numbers and descriptions of consultation content, effectively guiding users to consult according to specific needs.
4. Increase service statistics in the service guidance column of the Website to monthly release statistics of online service data.
5. As of December 31, 2020, there were 47 columns set on the Chinese website, including 6 dynamic news columns, 13 announcement information columns, 1 column for policies and regulations, 8 columns for data query, 9 columns for special topics, 1 column for institutional functions, 4 interactive exchange columns and 5 service columns. There were 6 columns set on the English website, including 3 dynamic news columns, 1 column for institutional functions and 2 columns for policies and regulations.
WeChat official account “CFDI Inspection Window” has three columns with 14 information categories, including 1 dynamic news column, 3 announcement information columns, 2 columns for policies and regulations, 4 columns for special topics, 3 columns for institutional functions, and 1 service column. WeChat official account had a total number of followers of more than 35,000 people from December 6, 2016 to December 31, 2020, in which, 4,519 people newly followed in 2020.

6. From January 1, 2020 to December 31, 2020, there were 3519 pieces of information actively released on the Chinese website, with an increase of 48% compared with that in 2019. Among them, there were 659 news releases, 2,846 announcements in key areas, and 14 pieces of information on policies, regulations and work procedures. There were 42 pieces of information released on the English website.
In 2020, both Chinese and English websites had 4.48 million views in total.
|
Category of Information Actively Released on the Chinese Website |
Quantity of Information (pieces) |
|
1. News announcement |
659 |
|
2. Announcements on key fields |
2846 |
|
3. Policies, regulations, and work procedures |
14 |
|
Total |
3519 |
|
Category of Information Actively Released on the English Website |
Quantity of Information (pieces) |
|
1.What's new |
29 |
|
2.Photo News |
9 |
|
3.Hot Topics |
4 |
|
Total |
42 |
7. From the date when the WeChat official account was created to December 31, 2020, a total of 613 messages were pushed and 1,324 pieces of information were released, with a total read quantity of 1.92 million times. In 2020, WeChat official account had pushed information for 130 times and released 227 pieces of information, with a read quantity of 360,000 times and an increase of 9.6% in quantity of information released compared with that of 2019.
Classified by the columns to which the information belonged, the quantity of information released in the main columns in 2020 was: 89 pieces for inspection dynamics, 52 for supervisory punishment, 30 for inspection announcement, 28 for inspection research, 10 for supervisory policies, 9 for inspection plan, 6 for comments seeking, 1 for technical guidance, 1 for annual report, and 1 for other information. Compared with 2019, the quantity of information on the latest progress of CFDI's inspection work was significantly increased in the inspection dynamics and inspection announcements.
|
Category of Information Released on the WeChat Official Account |
Quantity of Information (pieces) |
|
1. Inspection dynamics |
89 |
|
2. Regulatory punishment |
52 |
|
3. Inspection announcement |
30 |
|
4. Inspection research |
28 |
|
5. Regulatory policies |
10 |
|
6. Inspection plan |
9 |
|
7. Comments seeking |
6 |
|
8. Technical guidance |
1 |
|
9. Annual report |
1 |
|
10. Others |
1 |
|
Total |
227 |
8. CFDI shall continue to maintain the existing database, including the GMP certification announcement, GAP inspection announcement for traditional Chinese medicinal materials, GLP certification announcement, clinical trial qualification inspection announcement and public data about GMP certification inspection. In 2020, there will be no new data in each database.
VI. Increase the efficiency to handle with complaints
(I) On-line consultation
In 2020, a total of 701 online consultation questions were received, in which, 655 were effective consultations, with a decrease of 7.5 % compared with that of 2019, and 46 were invalid consultations due to the incomplete or unclear description of the problems. In the effective consultations, 470 were replied, with a response rate of 72% and a 38.5% increase compared with that of 2019. Among the replied questions, 61 common questions (or problems with high attention) related to drug production and the business of CFDI had been published on the website, 388 general questions had been replied by email or SMS, and other questions had been further communicated with consultants and relied by telephone.
(II) On-line complaints and handling
In 2020, a total of 26 complaints were received, an increase of 37% compared with that of 2019. Among these complaints, 21 complaints were out of the functional scope of CFDI and had been transferred to the Center for Administrative Services and Complaints&Reporting of NMPA for handling; 2 were consultation letters from the public and 3 were related to inspection items of CFDI, which had been transferred to the business department of CFDI for handling. The handling rate of complaints and reporting was 100%.
(III) Online appointment
In 2020, a total of 148 online appointments were received. CFDI timely processed the received information and distributed the appointment information to all offices to complete on-site communication.
(IV) On-line investigation
The online questionnaire survey column on the website of CFDI is used to collect public opinions and suggestions on the website to improve the service quality of information disclosure and other work of the website. As of December 31, 2020, CFDI had received two valid questionnaire feedbacks, which had been transferred to relevant departments of CFDI and handled in accordance with the Procedures for Management of Satisfaction Survey .
Ⅶ. Main problems and improvement measures
In 2020, in the face of new requirements of drug inspections for prevention and control of Novel Coronavirus epidemic and in the face of new demands of the public for drug inspection information, CFDI steadily pushed forward the construction of the platform and information disclosure, and achieved good results in the construction online office service platform, joint inspection information disclosure for drug registration, replies to online consultation, etc. However, there is still a gap compared with the requirements of the NMPA and the needs of the public.
In 2021, according to the Regulation of the People's Republic of China on the Disclosure of Government Information and relevant requirements of the NMPA, CFDI will focus on the following work:
(I) Continue to strengthen the construction of the information disclosure platform. In accordance with the need for on-site inspections, CFDI shall continue to build and complete the “Window for Drug Registration Applicants” based service platform, improve the interactive experience of applicants on the online service and the information disclosure function of the platform, and enhance the informationalized level of inspections.
(II) Continue to promote information disclosure. In accordance with the requirements of new laws and regulations and in close combination with the changes in the functions of CFDI, the columns on the website shall be adjusted to strengthen the publicity of drug inspection policies and to meet the public’s demand for information around the Drug Administration Law , Vaccine Administration Law , Administrative Measures for Drug Registration , Measures for the Supervision and Administration of Drug Production and other laws and regulations.
(III) Improve information disclosure management. CFDI shall strengthen the management of the content of information released, strictly implement the procedures for information disclosure, and improve the service level of information disclosure.
Center for Food and Drug Inspection of NMPA
March 26, 2021


