2022 Annual Report on Information Disclosure
In accordance with relevant provisions of the Regulations of the People's Republic of China on the Disclosure of Government Information and in combination with the actual situations of information disclosure by the Center for Food and Drug Inspection of NMPA (hereinafter referred to as CFDI) in 2022, this annual report has been formulated. This report covers an overview, system construction, disclosure of key inspection information, optimization of the online service platform, construction of website and new media platform, consultations and complaints & reports, issues and improvements, and other aspects. The data listed in this report was summarized from January 1, 2022 to December 31, 2022.
If you have any questions about this report, please contact the Information Management Division of CFDI (Address: Floor 7, Beikuang Finance Building, Building 3, Yard 1, Wenxing Street, Xicheng District, Beijing; Postal Code: 100044; Tel: 010-68441191).
I. Overview
In 2022, CFDI improved the information disclosure system, adjusted columns for key inspection business, optimized service platforms, and constantly improved the quality of information disclosure according to the Regulations of the People's Republic of China on the Disclosure of Government Information and the Notice on Website Evaluation of Directly Affiliated Institutions in 2022 issued by the News and Publicity Center of NMPA.
II. Improvement of the Information Disclosure System
In 2022, CFDI further improved the information disclosure management system. In addition to revising the Management Procedures for Information Release on the Website of CFDI and the Management Procedures for Information Release on the WeChat Official Account of CFDI , CFDI has also studied and formulated the key points of review for information release, refined the information disclosure process, and strengthened the review of important information to ensure the accuracy, timeliness, standardization, and effectiveness of information disclosure and release.
III. Adjustment of Information Columns for Key Inspection Business
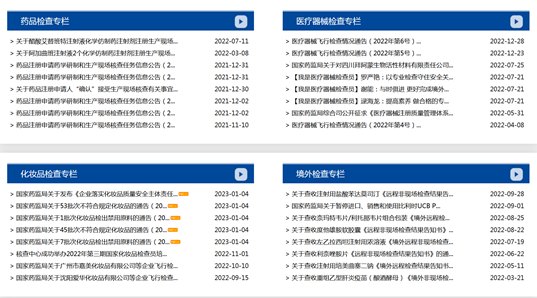
In 2022, CFDI adjusted the columns for key inspection business to four special columns, including the Special Column for Drug Inspection, the Special Column for Medical Device Inspection, the Special Column for Cosmetics Inspection, and the Special Column for Overseas Inspection, with 50 pieces of information released in total.
|
Information Category of Key Inspection Business |
Quantity of Information (Piece) |
|
1. Special Column for Drug Inspection |
2 |
|
2. Special Column for Medical Device Inspection |
16 |
|
3. Special Column for Cosmetics Inspection |
21 |
|
4. Special Column for Overseas Inspection |
11 |
|
Total |
50 |
IV. Optimization of the Online Service Platform
In 2022, CFDI continuously optimized and improved the security and convenience of the online service platform. With the addition of the login method by a digital certificate to the "Window for Drug Registration Applicants", applicants can select the login method by account ID and password or by a digital certificate as needed, which has not only strengthened information security management and improved the website security protection level but also reduced the burden of drug registration applicants on account ID management on multiple platforms. As of December 31, 2022, a total of 2,610 users have registered on the platform of the "Window for Drug Registration Applicants", among which a total of 989 users have bound their account IDs with digital certificates.
V. Improvement of Website and New Media Platform Columns
(I) Construction of Special Topics and Columns
1.CFDI has launched special columns for the "20 th CPC National Congress" and for "Deeply Studying and Implementing the Spirit of the 20 th CPC National Congress" to reprint information about the 20 th CPC National Congress on the Chinese Government Website (www.gov.cn) and the official website of NMPA.
2.CFDI has launched a "Special Column for Drug Inspection" to release information and trends related to drug inspection.
3.CFDI has additionally launched a column for "Notice and Announcement" to release the latest notice and announcement information in a prominent position on the homepage.
(II) Column Adjustment
1. CFDI has adjusted and integrated the columns for "Policies and Regulations". Specifically, CFDI has integrated the columns for "Policies and Regulations for GMP for Drugs", "Policies and Regulations for GAP for Traditional Chinese Herbal Medicine", "Policies and Regulations for GLP for Drugs", "Policies and Regulations for GCP for Drugs", "Policies and Regulations for GCP for Medical Devices", "Policies and Regulations for GMP for Medical Devices", "Policies and Regulations for On-site Inspection of Registration and Production of Drugs", and "Policies and Regulations for GSP for Drugs" into the following three categories for the sake of facilitating classified access and browsing: "Policies and Regulations for Drugs", "Policies and Regulations for Medical Devices", and "Policies and Regulations for Cosmetics".

2.CFDI has adjusted some columns with low update frequency and with updates stopped due to the end of relevant events to "New Columns" and "Historical Columns", in which the "Special Column for On-site Inspection of Registration and Production of Chemical Generic Injections", the "Special Column for On-site Inspection of Pharmaceutical Development and Production", and the "Special Column for Inspection of Clinical Trial Data" have been adjusted to the "Special Column for Drug Inspection", and the "ICH Guidelines", "Guidelines", and "Consistency Evaluation" have been adjusted to "Historical Columns". CFDI has canceled the columns with repeated contents, including the "Special Column for Unannounced/Tracking Inspection" and the "Special Column for Inspection Plan".
3.CFDI has further standardized column tags, including sorting out keywords for column tags and modifying them to keywords the same as those described in the columns.
(III) Website-related Data
As of December 31, 2022, a total of 24 columns have been launched on the Chinese website, including 9 columns for news and highlights, 1 column for policies and regulations, 4 special columns for special topics, 1 column for institutional functions, 4 columns for interactive exchanges, and 5 columns for services. A total of 6 columns have been launched on the English website, including 3 columns for news and highlights, 1 column for institutional functions, and 2 columns for policies and regulations.
In 2022, a total of 773 pieces of information were actively disclosed on the Chinese website, including 704 pieces of information about news and announcements, 50 pieces of information about the key inspection business, and 19 pieces of information about policies, regulations, and working procedures. A total of 19 pieces of information were released on the English website.
In 2022, the Chinese and English websites received 3.4 million page views in total.
|
|
|
|
Homepage of Chinese Website |
Homepage of English Website |
|
Category of Information Actively Disclosed on Chinese Website |
Quantity of Information (Piece) |
|
1. News and Announcements |
704 |
|
2. Key Inspection Business |
50 |
|
3. Policies, Regulations, and Working Procedures |
19 |
|
Total |
773 |
|
Category of Information Actively Disclosed on English Website |
Quantity of Information (Piece) |
|
1. What's New |
11 |
|
2. Laws & Regulations |
5 |
|
3. Photo News |
2 |
|
4. Hot Topics |
1 |
|
Total |
19 |
(IV) Data on WeChat Official Account
The WeChat Official Account "CFDI Inspection Window" has 14 information categories, including 1 column for news and highlights, 3 columns for announcement information, 2 columns for policies and regulations, 4 special columns for special topics, 3 columns for institutional functions, and 1 column for services. As of December 31, 2022, the total number of followers has reached more than 49,000.

In 2022, the "CFDI Inspection Window" pushed information 79 times and released 124 pieces of information, with a reading quantity of 240,000 times.
|
Category of Information Disclosed on WeChat Official Account |
Quantity of Information (Piece) |
|
1. Inspection Trends |
68 |
|
2. Regulatory Punishment |
14 |
|
3. Regulatory Policies |
12 |
|
4. Opinion Solicitation |
9 |
|
5. News and Highlights |
7 |
|
6. Inspection Announcements |
6 |
|
7. Inspection Research |
5 |
|
8. Technical Guidance |
2 |
|
9. Annual Report |
1 |
|
Total |
124 |
VI. Increase the Efficiency of Handling Complaints & Reports
(I) Online Consultation
In 2022, CFDI received and handled a total of 739 online consultations, with a handling rate of 100%. Among them, there were 720 effective consultations, with an increase of 13.6% over 2021; there were 19 ineffective consultations, with the main reason stating that they were incompletely or unclearly described. A total of 626 effective consultations have been replied to, with the rest under reply and review. Among the questions that have been replied to, 61 common questions (or questions with high attention) related to the inspection business have been released on the website, general questions have been replied to those who ask for consultations by email or SMS, and other questions have been further communicated with and replied to those who ask for consultations by telephone.
(II) Complaints & Reports and Handling
In 2022, CFDI received a total of 38 complaints & reports (17 repeated ones). Among them, 5 complaints were out of the functional scope of CFDI and had been transferred to the Center for Administrative Services and Complaints & Reports of NMPA, and the Division of General Affairs (Office of Public Complaints and Proposals) of the Department of Comprehensive Affairs, Planning, and Finance Affairs for handling. 11 were for consultation from the Division of General Affairs (Office of Public Complaints and Proposals) of the Department of Comprehensive Affairs, Planning, and Finance Affairs, 2 were transferred from the Department of Drug Registration, 2 were transferred from the Center for Drug Evaluation, and 1 was from the masses, which had all been transferred to the Business Department of CFDI for handling. The handling rate of complaints & reports was 100%.
(III) Online Appointment
In 2022, CFDI received and timely handled 29 online appointments. Specifically, CFDI distributed these appointments to relevant divisions and completed on-site communication.
(IV) Online Investigation
The Online Questionnaire Survey Column on the website of CFDI is used for the collection of public opinions and suggestions on the website to improve the service quality of information disclosure and other work of the website. As of December 31, 2022, CFDI has received 10 effective questionnaire feedbacks and transferred them to its relevant departments for handling according to the Procedures for Management of Satisfaction Survey .
VII. Main Issues and Improvement Measures
In 2022, CFDI continued to promote the disclosure of drug inspection information and achieved good results in the construction of the online service platform, reply to online consultations, etc. However, CFDI still failed to fully meet the requirements of NMPA and the needs of the public. The disclosure of key inspection information should be further intensified.
In 2023, CFDI will fully implement the spirit of the 20 th CPC National Congress and focus on the following work in strict accordance with the Regulations of the People's Republic of China on the Disclosure of Government Information and relevant requirements of NMPA: (I) Further intensify information disclosure, specifically intensify the disclosure of key inspection information according to the requirements of laws and regulations in combination with the functions of CFDI, and strengthen the publicity and interpretation of inspection policies. (II) Continue to improve the construction of such information disclosure platforms as the website, WeChat Official Account, etc., and optimize and improve column settings to meet the public's demand for information access. (III) Improve the working mechanism of information disclosure, strengthen the content management of information released, strictly implement information release procedures, and improve the information release quality.
Center for Food and Drug Inspection of NMPA
January 5, 2023